Click on a header on the header panel above to get directed to the main sections of this documentation
To navigate through more specific sections of this documentation, scroll through the grey navigation panel present at the right side of this page and click on a header.
To get a quick idea of how you can select the most relevant results from ORVAL, click on the Tutorials header above.
Summary
ORVAL is the first web bioinformatics platform for the exploration of predicted candidate disease-causing variant combinations, aiming to aid in uncovering the causes of oligogenic diseases (i.e. diseases caused by variants in a small number of genes). This tool integrates innovative machine learning methods for combinatorial variant pathogenicity prediction, further external annotations and interactive and exploratory visualisation techniques.
What can you do with ORVAL?
SUBMIT AND FILTER YOUR VARIANTS
You can submit the variants of a single individual either as a
variant list or a
VCF file.
You can also filter your variants based on their Minor Allele
Frequency (MAF), their position in the gene and/or based on
a specific gene panel of your choice.
PREDICT CANDIDATE DISEASE-CAUSING VARIANT COMBINATIONS
With ORVAL you can predict candidate pathogenic variant combinations in any gene pair present in your data with VarCoPP and further predict their digenic effect (True Digenic, Monogenic with a Modifier variant or Dual Diagnosis) with the Digenic Effect Predictor.
EXPLORE POTENTIAL OLIGOGENIC SIGNATURES
You can investigate potential oligogenic disease signatures by exploring the interactive gene networks that are created based on the predictions and examine them in the context of their protein-protein interactions, cellular locations and pathways.
NOTE: The main results of this platform are based on predictive tools. They are provided for research,
educational and informational purposes only and the pathogenicity predictions should be subject to further scientific and clinical investigation.
It is not in any way intended to be used as a substitute for professional medical advice, diagnosis, treatment or care.
The input data
ORVAL accepts a list of variants from a single individual only, as it creates all possible variant combinations between pairs assuming that these belong to the same individual.
You can provide either Single Nucleotide Variants (SNVs) or small insertions/deletions (indels).
NOTE: The use of ORVAL for the analysis of complete patient exomes is NOT recommended, as our methods are not fine-tuned for exomes. Please restrict your analysis to relevant gene panels for the disease of interest. If your VCF contains the complete exome of an individual, you can upload it in ORVAL as it is, and then use the Variant Filtering and Gene Filtering options before submitting your data.
Types of input files
There are two different types of variant input that you can use to upload your data: either a variant list or a VCF file. After uploading your data, you can start the analysis by clicking on the button.
Tab-delimited variant list
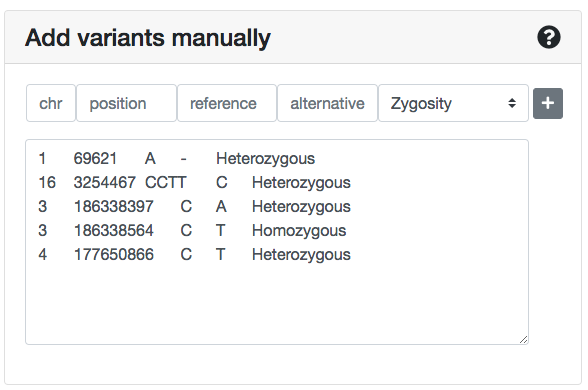
At the left panel of the Submit variants page you can insert/copy-paste a variant list. Each line should contain tab- or space-delimited information for one variant, in the corresponding order: chromosome, position, reference allele, alternative allele, zygosity.
No headers are needed.
The zygosity values should be either Heterozygous or Homozygous. During the analysis, ORVAL automatically converts X-linked variants in males as Hemizygous.
You can also manually insert a variant in this list by typing information:
- at the next line and making sure that you use the same delimiter for all columns
- at the corresponding chr, position, reference allele, alternative allele, Zygosity column fields and pressing the button.
NOTE: using the variant list panel, you can upload up to 80000 variants.
VCF file
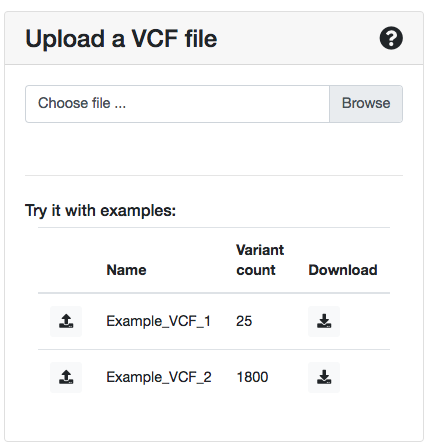
Alternatively, you can submit a VCF file (version 4.2) with your variants at the right panel of the submission page.
ORVAL requires as minimum the presence of:
- the #Header Line: #CHROM POS ID REF ALT etc... line
- the columns CHROM, POS, ID, REF, ALT, FORMAT, SAMPLE_NAME (patient information column containing values corresponding to
the FORMAT field).
- the genotype (GT) field for each variant at the
FORMAT and SAMPLE_NAME columns.
We use GT to define the zygosity of the variant and to pick the correct alternative allele if there are multiple ones (separated by commas) in ALT.
In case variants with GT: 0/0 ; 0|0 ; ./. ; . are present, these are discarded from the analysis. Allele combinations only consisting of alternative alleles (e.g. GT: 1/2 or 3/2) are handled as two separate heterozygous variants.
Any other meta-information lines on the top of the file or any extra columns and fields (e.g. QUAL, INFO, etc.) can be present, but ORVAL will ignore them.
NOTE: if your VCF contains information for several individuals, you should separate the information of each individual in different VCF files and run them individually in ORVAL.
NOTE: you can upload a VCF file of size up to 50 MB. The file can also be compressed either with zip, gzip, bzip2 or xz.
In case you want to create your own VCF file, you can download and take a look at the example VCFs that are present at the VCF submission panel and/or consult the Samtools specification page on how to construct a proper VCF file.
Variant types
You can either submit Single Nucleotide Variants (SNVs) or small insertions/deletions (indels). Other types of variants (e.g. CNVs) can be present in your list, but they will not be included in the analysis.
Specifically for indels, you can submit your variants in either one of the two different ways that are shown for a particular variant example (the VCF file can contain more columns).
| Tab-delimited list | VCF file | |
|---|---|---|
| Example with dashes | 16 3254468 CTT - Heterozygous | 16 3254468 . CTT - PASS GT 1/0 |
| Example without dashes | 16 3254467 CCTT C Heterozygous | 16 3254467 . CCTT C PASS GT 1/0 |
Genome version
At the moment ORVAL accepts and annotates variants using either the GRCh37/hg19 or the GRCh38/hg38 human genome assembly.
We do not make conversions of genomic coordinates from different genome versions. In case you need to convert your variants, you are encouraged to use tools like the UCSC, Ensembl and NCBI assembly converters.
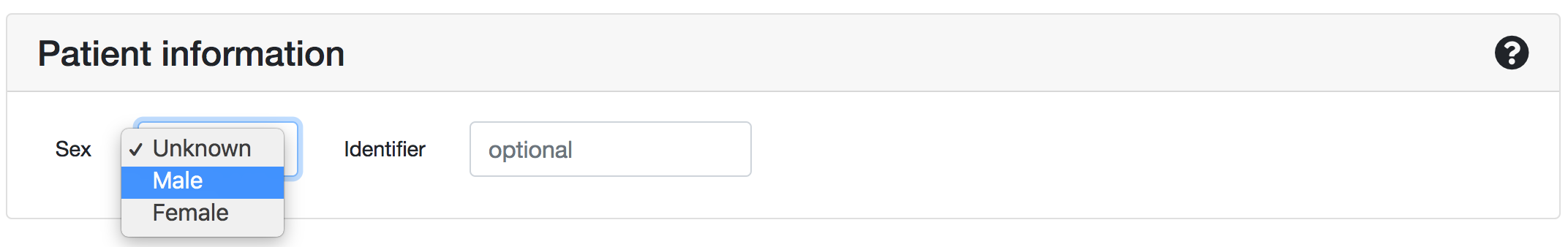
Patient information
Except from the variant list, you should also provide (if available) the sex information of the patient, i.e. if the person is a male or a female.
ORVAL handles differently X-linked variants in males (hemizygous variants) compared to females, and therefore this information is important in order to provide better predictions.
Example input files
You can try ORVAL with the two example VCF files that are present in the VCF file section of the variant submission page (for both genome assemblies). These files give you the opportunity to test ORVAL on a small or large number of variants and see what the webserver has to offer.
- the Example_VCF_1 file contains 25 variants.
- the Example_VCF_2 file contains 1800 variants.
Job submission
Every time you submit your data, you will first get directed to the Submitted ORVAL Job page where you can follow the status of your submission.
In this page you will also receive a Job Id, which you can use to re-access the results of that specific submission or report errors. That Job Id is also present in the Results site in the format: orval.ibsquare.be/results?id=YourJobID.
You can re-access your results by:
- saving the URL of the Job or the Results page
- typing on your browser https://orval.ibsquare.be/results?id= followed by the Job ID
Do you receive error or warning messages during your data submission? You can consult the Frequently Asked Questions (FAQ) section for suggestions on how to handle them.
NOTE: all result information is automatically deleted 7 days after the submission at 04.00 am (GMT+2). After this period, you have to re-submit your data and you will receive a new Job Id.
NOTE: for server monitoring purposes we allow every user (based on their IP address) to run up to 5 different Submission Jobs at the same time. In case you exceed this number, you will have to wait until at least one of the running Jobs is finished to launch a new one.
Data filtering and annotation
Data filtering
In the Submit variants page, ORVAL offers a recommended variant and gene filtering procedure that will automatically run when you submit your data. This procedure is highly recommended, as it will limit the amount of variant combinations to be tested and will restrict the analysis to the most relevant variants.
There are two types of filtering offered by ORVAL: a variant filtering and a gene filtering procedure.
Variant filtering
The variant filtering procedure ensures that your analysis will contain variants in accordance with the variant types used to train the predictive methods (VarCoPP and Digenic Effect predictor) integrated in ORVAL: exonic and splicing variants of MAF lower or equal than 3.5% in protein-coding genes.
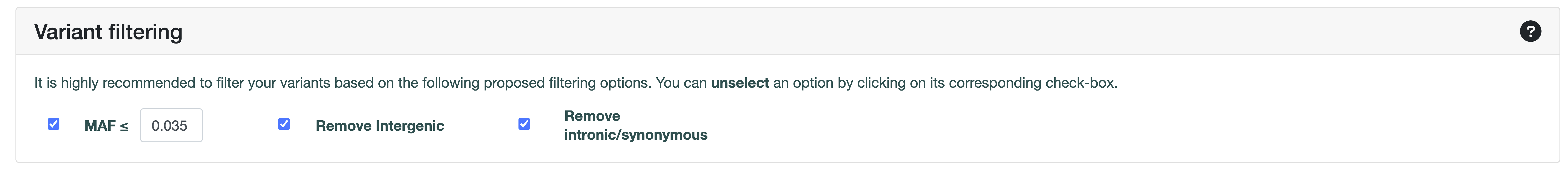
The three different filtering options are already pre-selected in the Variant Filtering panel of the submission page. You can unselect a filtering option, by clicking on its corresponding check-box.
Filtering options
MAF
Select the minimum threshold of MAF for the variants. A MAF of ≤ 0.035 was used to train VarCoPP and is the recommended threshold.
Remove Intergenic
Removes variants that are not inside the defined gene coordinates, based on the selected assembly (GRCh37/hg19 or GRCh38/hg38).
Remove intronic and synonymous
Removes:
- all intronic variants that have a distance bigger than 13 nucleotides from each exon edge, based on the exon coordinates of the canonical transcript of the gene.
- all synonymous variants that have a distance bigger than 195 nucleotides from each exon edge, based on the exon coordinates of the canonical transcript of the gene.
NOTE: apart from the requested filtering steps, ORVAL may also exclude some extra variants during the data annotation process. You can consult the complete list of variant exclusion cases during that process here.
Gene filtering
The gene filtering option restricts the analysis to a specified list of relevant genes that can be present in your data. This procedure is highly recommended in case your VCF contains the complete exome of an individual, as it can dramatically limit the amount of False Positives that can be obtained.
To run your analysis only with a subset of genes, you can simply upload a .txt file with the genes you are interested to include, each gene being in a different line. You can submit ENSGs or gene names. After submission, ORVAL will use this list to filter the genes that will be used in the analysis.
NOTE: please make sure that you provide the official approved symbol or ENSG for each gene (for verification, you can consult the HGNC gene nomenclature page or the Ensembl genome browser.
Data annotation
After you submit your data, ORVAL:
- automatically annotates them with the biological information (list of sources and current versions in the link) needed for the integrated predictive methods (VarCoPP and the Digenic Effect predictor)
- creates all possible variant combinations between any pair of genes present in your variant input and
- orders the variants and genes inside each combination.
Below, you can find some important parameters for each process.
Gene annotation
To map variants in genes, ORVAL uses at first the gene information that is present in the CADD annotation file for that variant and uses only the canonical transcripts of those genes, according to the Ensembl GRCh37/hg19 or GRCh38/hg38 genome version.
In cases where a variant can be mapped to multiple genes, ORVAL maps that variant to only one gene based on a set of priority rules that include (starting from higher to lower priority): valid gene IDs and canonical transcript, prioritisation of genes based on their biotype and the functional consequence of the variant, prioritisation of genes where the variant falls inside the gene and canonical transcript coordinates, presence of a CCDS, prioritisation of gene with the longest canonical transcript, etc.
ORVAL then annotates the genes with:
- the required gene features for VarCoPP (detailed description of the features and their sources in the link)
- the required gene features for the Digenic Effect predictor (detailed description of the features and their sources in the link)
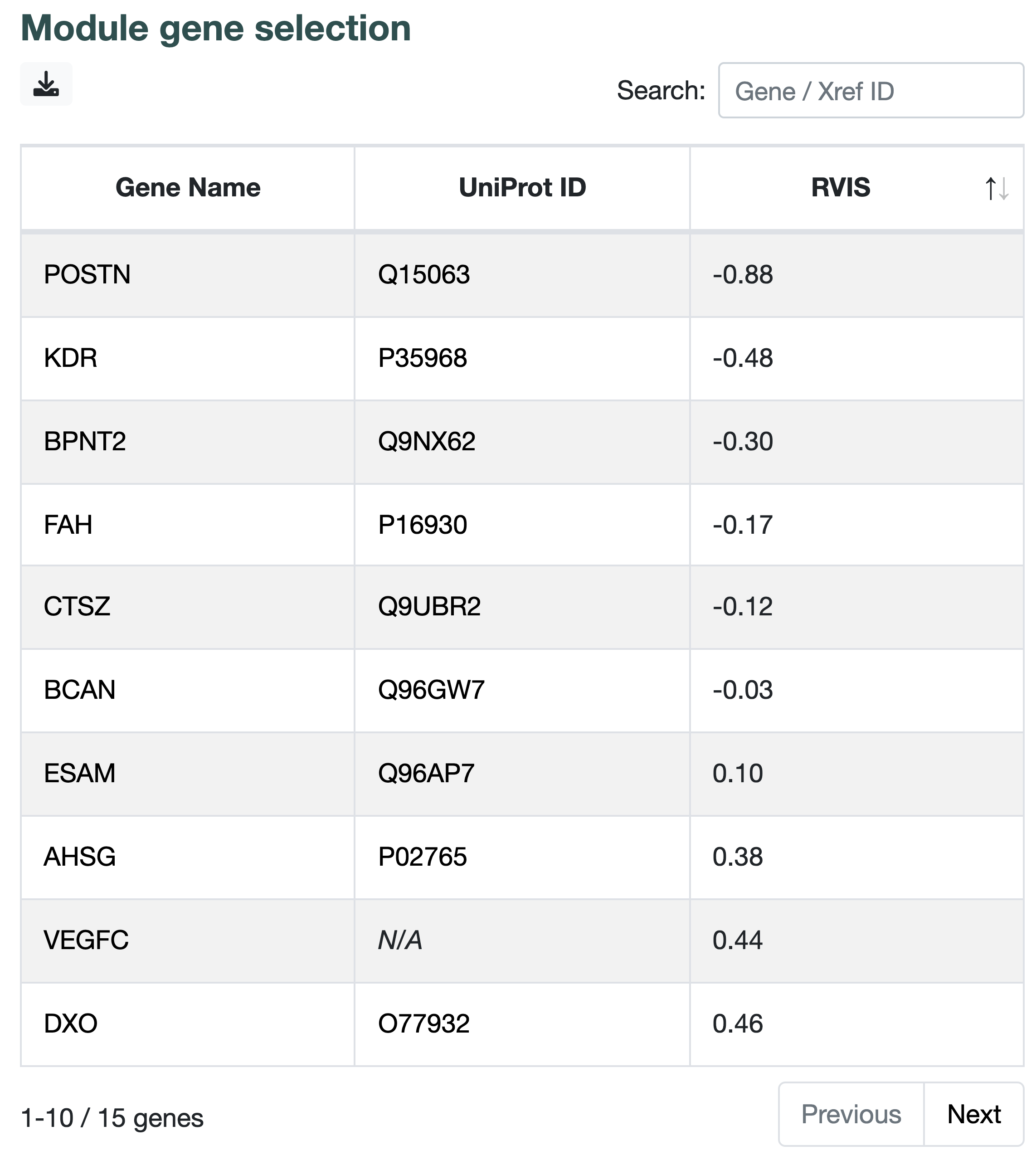
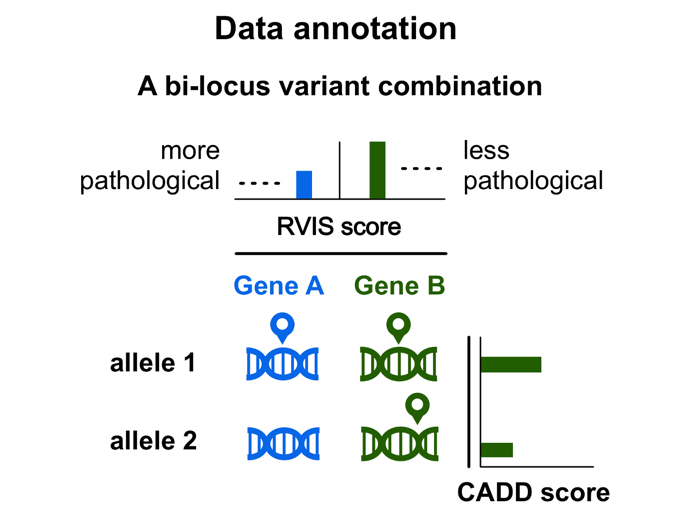
- the Residual Variation Intolerance Score (RVIS), a metric that show the susceptibility of a gene to disease. Lower values of RVIS indicate greater susceptibility of a gene to candidate disease-causing mutations.
- the protein sequences from Uniprot using the Ensembl canonical identifiers, as these are needed to calculate some of the features of our predictive methods
Gene pair annotation
ORVAL annotates a gene pair with:
- the gene pair features required by VarCoPP (detailed description of the features and their sources in the link).
- involvement in the same pathway information from Reactome, which is used as a feature for the Digenic Effect predictor
- protein-protein interaction (PPI) and cell co-localisation information from the comPPI database
ORVAL uses the Residual Variation Intolerance Score (RVIS) metric to order the appearance of genes inside each digenic variant combination, with gene A being always the gene with the lower RVIS value, and thus more probable to be have a disturbed function due to the presence of a variant. You can find more details about how ORVAL creates digenic variant combinations and orders variants and genes in the Creating digenic variant combinations section of the Documentation page.
Variant annotation
ORVAL first maps a variant in a gene based on the Gene annotation process described above. It then annotates each variant with:
- the required variant features for VarCoPP, the most important being the CADD score (detailed description of the features and their sources in the link)
- the required variant features for the Digenic Effect predictor (detailed description of the features and their sources in the link)
When ORVAL creates digenic variant combinations, it uses the CADD score to order the appearance of variants that are present inside the same gene (i.e. in cases of heterozygous compound variants). You can find more details about how ORVAL creates digenic variant combinations and orders variants and genes in the Creating digenic variant combinations section of the Documentation page.
Variant exclusion
In some situations during the data annotation process ORVAL excludes variants from the analysis and you will not find them in the results:
- Variant not exonic in canonical transcript
We use only the canonical Ensembl transcript identifiers to annotate our variants. If you have selected to exclude intronic variants from your analysis, if the variant is not exonic in the canonical transcript of the gene, even if it may be exonic in an alternative transcript, it will be excluded. - Variant with invalid zygosity
Variants with GT:0/0 or GT:0|0 in a VCF file are considered invalid and are excluded from the analysis. - CADD score not available
ORVAL annotates variants with a CADD score, which is a feature required for the pathogenicity predictions. As this feature is important for the predictions, if a CADD score is not available for a variant, that variant is excluded for the analysis, as a missing value may severely alter the results. - Variants only in one gene
As ORVAL creates combinations between gene pairs, if your input data includes variants from one gene only, you will not get any results. - The variant is a CNV or medium/long-sized InDel
ORVAL analyses only SNVs and small insertions and deletions (up to 100 bp currently). Any other variant type in your data is automatically excluded from the analysis. This is mainly due to the computational resource limitations of our service.
Creating digenic variant combinations
After annotation, VarCoPP creates all possible variant combinations between any gene pair present in your input, taking into consideration any filtering options you have included during your variant submission.
You can find below a list of details and constraints that take place during this procedure.
Number of variants per combination
ORVAL creates for any gene pair variant combinations that can be:
- bi-allelic (i.e. one mutated allele at each gene)
e.g.: one heterozygous variant per gene - tri-allelic (i.e. three mutated alleles in total)
e.g.: an homozygous variant at gene A and an heterozygous variant in gene B - tetra-allelic (i.e. four mutated alleles in total)
e.g.: one homozygous variant per gene
In the tri-allelic and tetra-allelic cases, a digenic combination can also include heterozygous compound variants (i.e. two different mutated alleles in the same gene), along with the presence of variant(s) in another gene.
NOTE: Tetra-allelic variant combinations with heterozygous compound variants in BOTH genes are not created.
NOTE: In case where multiple heterozygous variants are present in a single gene,
these are used together as heterozygous compound variants in a combination with another gene and not individually anymore.
This is to solve the problem of certain genes being over-represented in the results just because they contain heterozygous compound variants.
If a single gene contains more than two heterozygous variants, these are used in pairs of two as we always use two mutated alleles per gene.
Order of genes
For each digenic variant combination, gene A is always the gene with the lowest Residual Variation Intolerance Score (RVIS) (see also the Gene Annotation section) and, thus, the one with a higher probability to be associated with a disease.
Order of variant alleles inside the gene
In case of two different mutated alleles in the same gene (heterozygous compound cases), the variant allele 1 is always the variant allele with the highest CADD score.
A graphical representation of a digenic combination
The predictive methods of ORVAL
VarCoPP: the variant combination pathogenicity predictor
VarCoPP stands for Variant Combination Pathogenicity Predictor. It is a machine-learning method that predicts the pathogenicity of any bi-locus variant combination (i.e. a combination of two to four variant alleles between two genes).
The method has been published in the PNAS journal: https://doi.org/10.1073/pnas.1815601116. The first version of the model was later improved to VarCoPP2.0 using new training data, new features and a different type of model. VarCoPP2.0 has been published in BMC Bioinformatics: https://doi.org/10.1186/s12859-023-05291-3. See also the Cite us section in the About page, for a list of all relevant citations.
Based on VarCoPP, a bi-locus variant combination can either be candidate disease-causing or neutral.
VarCoPP has been trained separately for the GRCh37/hg19 and GRCh38/hg38 genome assembly, the correct model will run based on your assembly choice in the Input section.
Structure of VarCoPP
ALGORITHM
VarCoPP2.0 is a Balanced Random Forest (RF) predictor that consists of 400 decision trees.
TRAINING DATA
The VarCoPP2.0 predictor has been trained on the pathogenic variant combinations present in the Oligogenic Diseases Database (OLIDA) against a large subset of variant data derived from control individuals of the 1000 Genomes Project (1KGP).
The variant types that were used for training were the same for both OLIDA and 1KGP: exonic and splicing variants of up to 3.5% MAF, while all genes were protein coding genes.
RESULT CALCULATION
VarCoPP2.0 outputs a VarCoPP score, i.e. the probability (value between 0 and 1) that a variant combination belongs to the disease-causing class. If this probability is above 0.50 (hg19) or 0.4575 (hg38), the model predicts that the combination is disease-causing.
Prediction features
VarCoPP uses different variant, genes and gene pairs biological features to make the predictions.
| Feature | Source | Feature abbreviation | Gene / Variant allele |
|---|---|---|---|
| CADD raw score
PMID: 24487276 |
CADD v1.6 | CADD1 CADD2 CADD3 CADD4 |
Gene A / Variant allele 1 Gene A / Variant allele 2 Gene B / Variant allele 1 Gene B / Variant allele 2 |
| Gene haploinsufficiency prediction
PMID: 28137713 |
dbNSFP v4.1 | HIPred_A HIPred_B |
Gene A Gene B |
| Inheritance specific pathogenicity prediction
for autosomal dominant (AD), autosomal recessive (AR), and X-linked (XL) modes of inheritance PMID: 27354691 |
publication supplementary data |
ISPP_AD_A ISPP_AD_B ISPP_AR_A ISPP_AR_B ISPP_XL_A |
Gene A Gene B Gene A Gene B Gene A |
| Selective pressure (dN/dS)
PMID: 26896847 |
Ensembl v99 | dN_dS_A | Gene A |
| Biological distance
PMID: 24694260 |
Human Gene Connectome v12.2015 |
Biol_Dist | Gene pair AB |
| Biological Process similarity
PMID: 10802651, 18460186 |
Gene Ontology v2021-12 |
BP_sim | Gene pair AB |
| Distance in an in-house developed Knowledge Graph
PMID: 37644440 | BOCK v1.0 |
KG_dist | Gene pair AB |
99% and 99.9% confidence zones
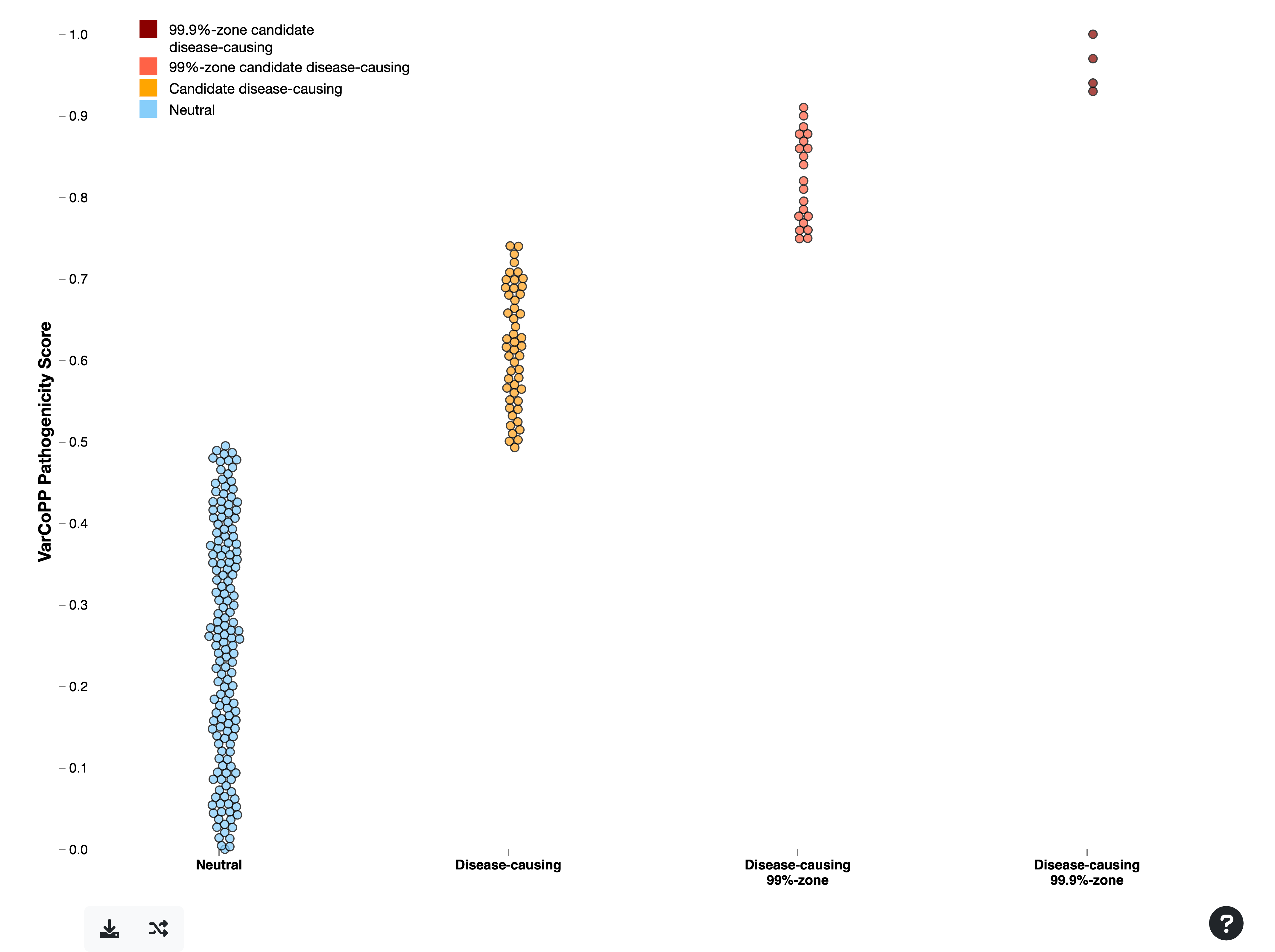
With VarCoPP2.0 we have defined 99% and 99.9%confidence zones, delimited by the prediction threshold, which provide a probability of whether a particular combination predicted as candidate disease-causing, is actually a True Positive (TP) result. This indication can be useful for further evaluation and filtering of the predictions.
These confidence zones were created by testing neutral bi-locus combinations from the 1000 Genomes Project and obtaining the minimal prediction probability that gave 1% and 0.1% False Positives respectively. If a combination falls into either one of the two zones, a coloured indication will appear in the summary results.
99%-confidence zone
Requires VarCoPP score ≥ 0.743 (hg19) or ≥ 0.647 (hg38). If a digenic combination falls inside this zone, it has 99% probability of being a TP result.
99.9%-confidence zone
Requires VarCoPP score ≥ 0.891 (hg19) or ≥ 0.85 (hg38). If a digenic combination falls inside this zone, it has 99.9% probability of being a TP result.
The Digenic Effect Predictor
The Digenic Effect predictor is a machine-learning method that predicts the type, or else the digenic effect of a pathogenic digenic variant combination. This information could be useful in case there is no pedigree information or parent genotypes available, as it could give a predictive indication of the effect of a predicted as pathogenic variant combination. As this is a machine-learning approach, again, a manual investigation by the user can confirm or reject the assigned digenic effect class.
The Digenic Effect predictor has been published in the Artificial Intelligence in Medicine journal: https://doi.org/10.1016/j.artmed.2019.06.006 and the Nucleic Acids Research journal: https://doi.org/10.1093/nar/gkx557. See also the Cite us section in the About page, for a list of all relevant citations.
IMPORTANT UPDATE: An update of the Digenic Effect predictor has been made using up-to-date versions of all required features (check the Updates page). This means that the obtained results may differ than those from the corresponding publication.
The Digenic Effect predictor can distinguish between three classes of pathogenic variant combinations:
True Digenic
Variants at both genes are needed to show the disease phenotype.
Monogenic + Modifier
The variant at the first gene acts as the major monogenic variant that can trigger disease symptoms, while the second variant acts as a modifier of symptoms severity or age of onset.
Dual Molecular Diagnosis
Conjunction of variants that trigger two independent monogenic disorders that occur simultaneously within a single patient.
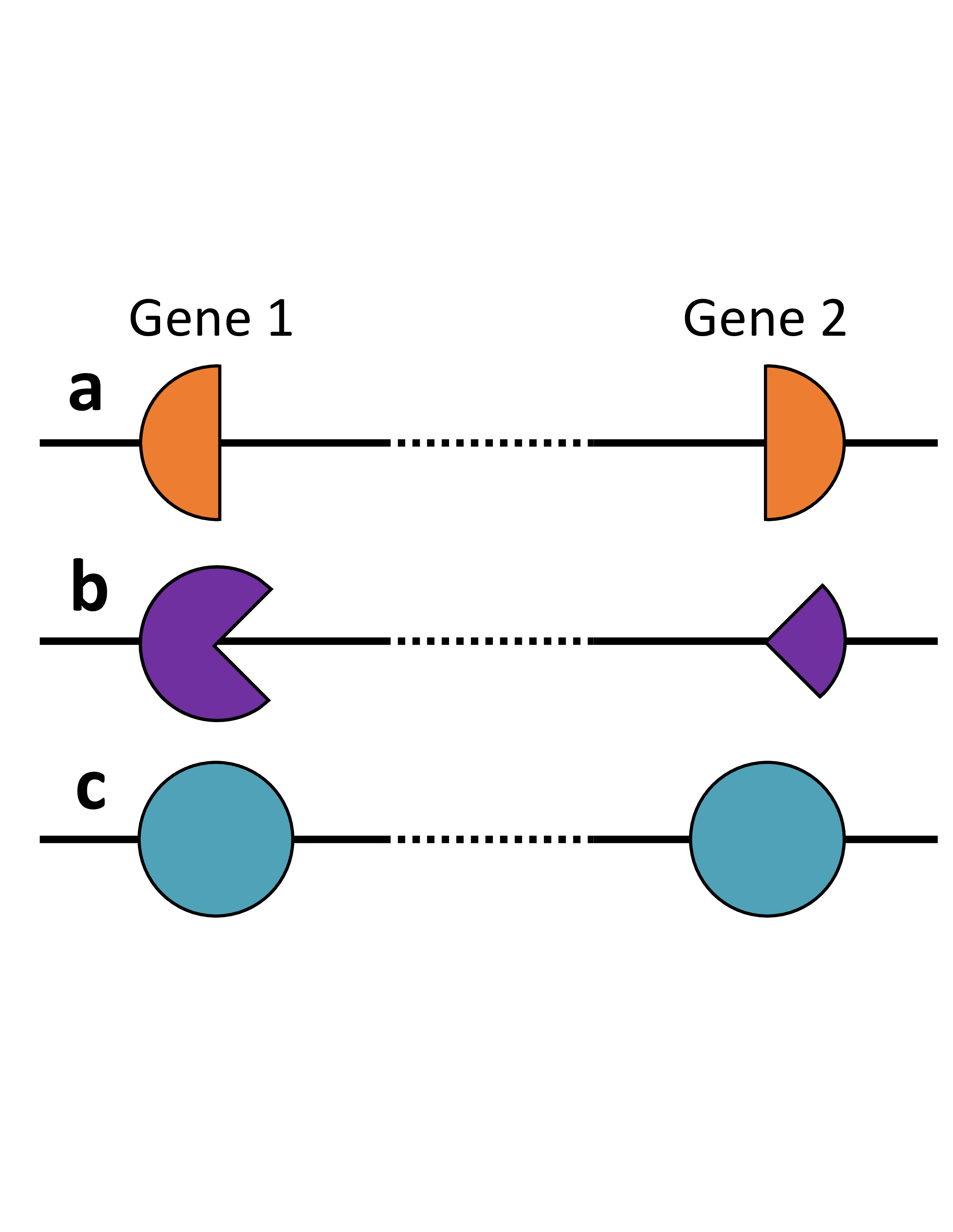
The three types of digenic effects.
Combination a, a True Digenic combination, where
the simultaneous presence of a pathogenic allele in each gene is necessary for the individual to express
the disease. phenotype.
Combination b, a Monogenic plus Modifier combination, where a variant on the major
gene induces a disease phenotype, while a mutation in the modifier gene modifies it, either by
rendering it more severe or producing an early onset.
Combination c, a Dual Molecular Diagnosis combination,
where both loci are responsible for either distinct or overlapping phenotypes for two different diseases.
The structure of the Digenic Effect predictor
ALGORITHM
The Digenic Effect predictor is a classification Random Forest (RF) algorithm.
TRAINING DATA
The Digenic Effect predictor was trained on 240 pathogenic variant combinations.
More specifically, it has been trained on 90 True Digenic and 75 Monogenic+Modifier variant combinations present in the Digenic Diseases Database (DIDA) and 75 Dual Molecular Diagnosis combinations derived from the work of Posey et al.
The variant types were single nucleotide variations and small insertions/deletions.
RESULT CALCULATION
The Digenic Effect predictor provides probabilities (from 0 to 1) for all three digenic effect classes for a variant combination.
The final digenic effect class is the class with the highest probability among the three.
Prediction features
The Digenic Effect predictor uses different variant, genes and gene pairs biological features to make the predictions.
| Feature | Feature abbreviation | Gene / Variant allele |
|---|---|---|
| CADD raw score
PMID: 24487276 |
CADD1 CADD2 CADD3 CADD4 |
GeneA / Variant allele 1 Gene A / Variant allele 2 Gene B / Variant allele 1 Gene B / Variant allele 2 |
| Gene recessiveness probability
PMID: 22344438 |
RecA RecB |
Gene A Gene B |
| Essential in mouse
PMID: 23675308 |
EssA EssB |
Gene A Gene B |
| Same pathway
SOURCE: Reactome |
Pathway | Gene pair AB |
HOP: High-throughput oligogenic prioritizer
HOP, the High-throughput Oligogenic Prioritizer, is a prioritization method that uses direct oligogenic information at the variant, gene, and gene pair level to effectively detect and rank digenic variant combinations in whole exome sequencing (WES) data. By combining specialized pathogenicity predictions with disease-relevance information, HOP helps clinicians identify the most likely disease-causing variant combinations in a patient's exome.
The method has been published in the Bioinformatics journal: https://doi.org/10.1093/bioinformatics/btae184 See also the Cite us section in the About page, for a list of all relevant citations.
Structure of HOP
How HOP works: Overview of the Prioritization Pipeline
HOP operates through a four-step process to rank variant combinations based on their likelihood of causing disease:
- Variant Combination Generation: All possible variant combinations between two genes are generated from the filtered VCF file, with a maximum of 2 variants per gene.
- Pathogenicity Scoring: Each combination is annotated with VarCoPP2.0 features and assigned a Pathogenicity Score (PS)—the probability that the combination is disease-causing.
- Disease-Relevance Scoring: A Disease-Relevance Score (DS) is computed for each gene pair by propagating disease information (HPO terms, gene panels, or both) through a knowledge graph using a random walk with restart algorithm.
- Final Ranking: Both scores are normalized and combined to produce a Final Score (FS), which ranks all variant combinations in the exome from highest to lowest likelihood of relevance to the disease.
Pathogenicity Score (PS)
HOP leverages VarCoPP2.0, a machine learning classifier trained on pathogenic variant combinations from the OLIDA database and negative controls from the 1000 Genomes Project. VarCoPP2.0 outputs a score between 0 and 1 representing the probability that a digenic variant combination is disease-causing.
Disease-Relevance Score (DS)
Knowledge Graph-Based Approach: HOP computes the Disease-Relevance Score (DS) by leveraging BOCK, a comprehensive heterogeneous knowledge graph containing information from 12 biological databases including protein-protein interactions, gene ontologies, pathways, and disease associations.
The DS is calculated using a Random Walk with Restart (RWR) algorithm, which:
- Propagates information from user-defined seed nodes (HPO terms, disease genes, or both) through the knowledge graph
- Outputs proximity scores for each gene based on their distance to the seed nodes
- Combines individual gene scores to generate a gene-pair DS as the average of both genes' scores
- Uses a restart parameter of 0.3 to balance local and global network topology
This approach is based on the principle of "guilt-by-association"—genes involved in the same disease tend to be functionally related and thus closer together in biological networks.
HOP combines the Pathogenicity Score (PS) and Disease-Relevance Score (DS) through a normalization and aggregation process
- Min-Max Normalization: Both PS and DS are normalized per exome such that the highest-scoring combination has a normalized score of 1 and the lowest has 0. This ensures equal weighting of both information types.
- Score Aggregation: The Final Score (FS) is computed as the average of
the normalized PS and DS:
FS = (PS_scaled + DS_scaled) / 2 - Ranking: All variant combinations in the exome are ranked by their FS in descending order, with the highest-scoring combinations appearing first.
Tutorials
In case you would like to see a specific tutorial in ORVAL, you can contact us.
Prioritisation of digenic variant combinations
Depending on the size of the data you are analysing you may end up with many digenic variant combinations predicted as candidate disease-causing. ORVAL is not a prioritisation tool per se, however, it is possible to limit your analysis to those combinations that could potentially be more interesting for your research. For this, you can follow the next steps:
- 1. Overview and filtering of the digenic variant combinations
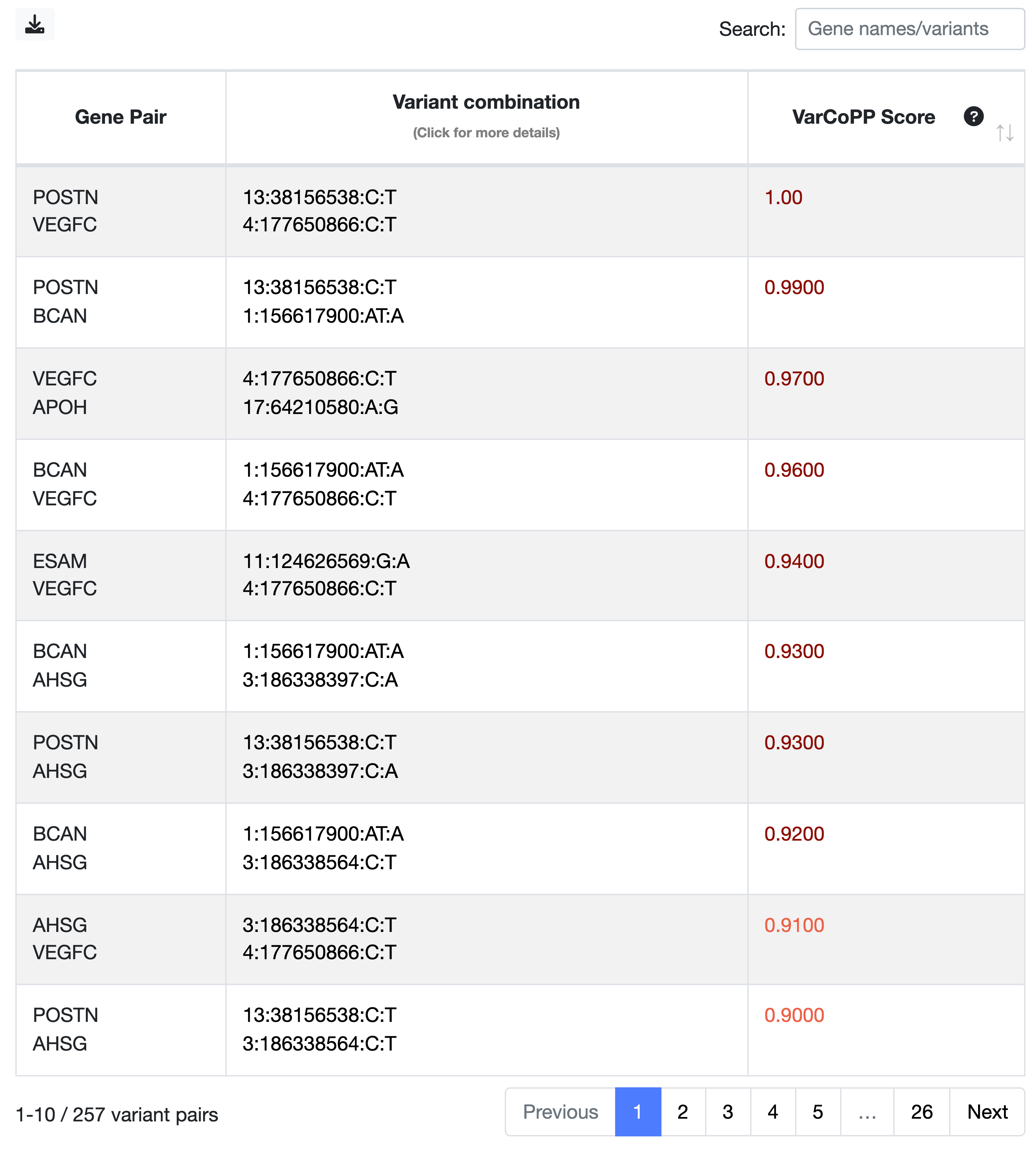
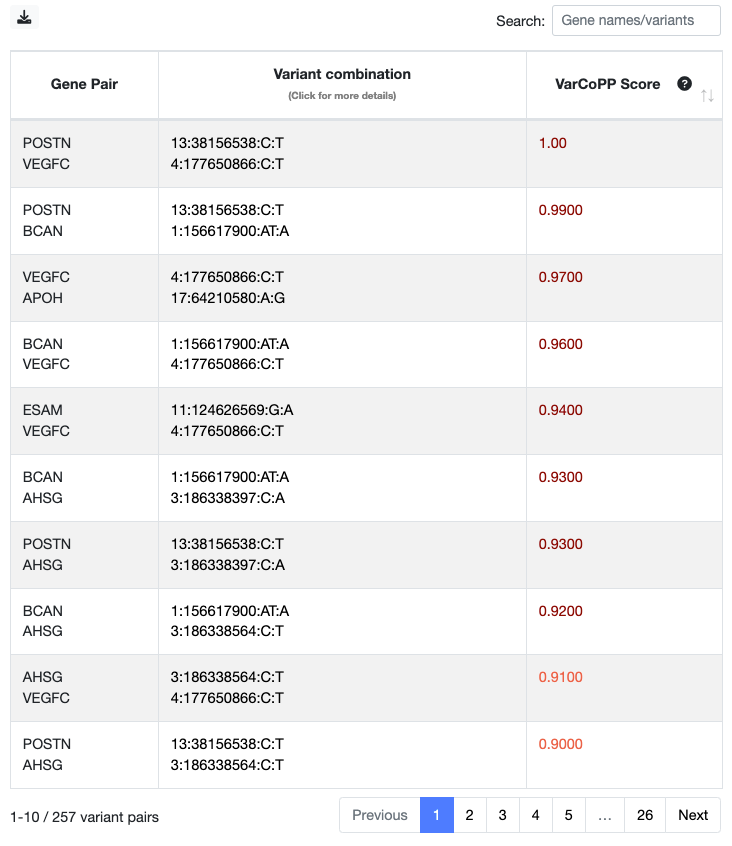
The combinations in the Summary Table are ranked based on their VarCoPP scores, with those at the top having the highest scores. The higher the score assigned to a digenic combination, the more confident VarCoPP is for the disease-causing class (consult the VarCoPP section for a detailed explanation).
- The strictest way to filter your combinations is by focusing on those falling in the 99.9%-confidence zone, in dark red colour (the first two combinations in this example). These have 0.1% probability of being False Positives. Keep in mind that this selection can be quite strict and can lead to False Negatives.
- To lessen your criteria, you can also include the combinations falling in the 99%-confidence zone in red colour (the next five combinations in this example), which have 1% probability of being FPs.
- If the steps described below do not yield convincing results, you can also try to include the combinations that are predicted as disease-causing but do not fall in any of the two confidence zones, and these are depicted in orange.
For more information, consult the VarCoPP Confidence Zones section of our Documentation page.
- 2. Find relevant gene modules in the predicted pathogenic gene network
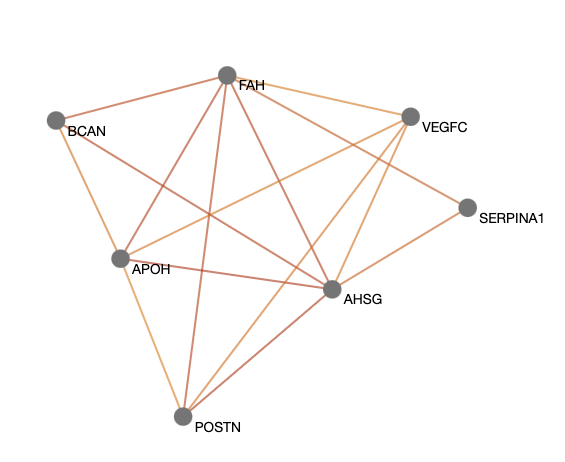
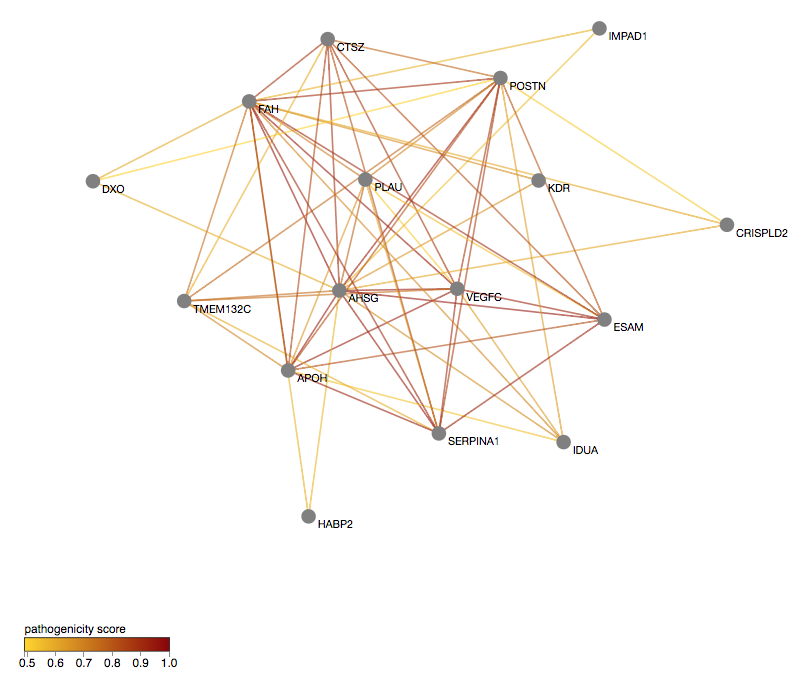
Are the genes relevant? You can explore the predicted pathogenic gene network in ORVAL, which appears first in the results page. In this network, two genes are connected with an edge only if they contain at least one predicted pathogenic digenic combination.
You can first filter the network to keep the most relevant gene pairs, based on the pathogenicity cutoff that you choose.
You can use the Filtering panel on the left, to keep only the gene pairs containing combinations falling in the 99%- and 99.9%-confidence zones,
by selecting 0.74 (hg19) or 0.65 (hg38) as the minimum Gene Pair Pathogenicity Score threshold (i.e., this is the minimum VarCoPP Score for the 99%-confidence zone, see
the VarCoPP Confidence Zones section of our Documentation page).
For a stricter analysis, you can use the 0.89 (hg19) or 0.85 (hg38) value as a cutoff to include only gene pairs with combinations falling in the 99.9%-confidence zone.
Once you are satisfied with your network, click on a gene in the network to make the gene module panel appear on the right and click on the link that appears to get directed to another page the shows PPI and pathway information for those genes.
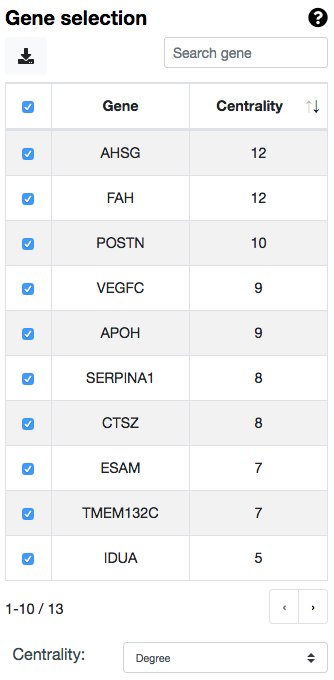
NOTE: if a gene is shown as a hub in the gene network, meaning that it is highly connected with other genes, it may mean that it contains a variant there that drives the predictions higher. This may indicate that a Monogenic plus Modifier concept may be present in your data. However, if this gene does not seem relevant for the phenotype or seems unrelated to the rest of the genes, you could try to remove it from the network and consider the rest of the genes. You can use a general Centrality threshold of your choice or unselect genes manually in the gene table on the left.
Detailed information on ways to filter your network can be found in the Oligogenic network section of our Documentation.
- 3. Explore the relationship between the genes in the gene module
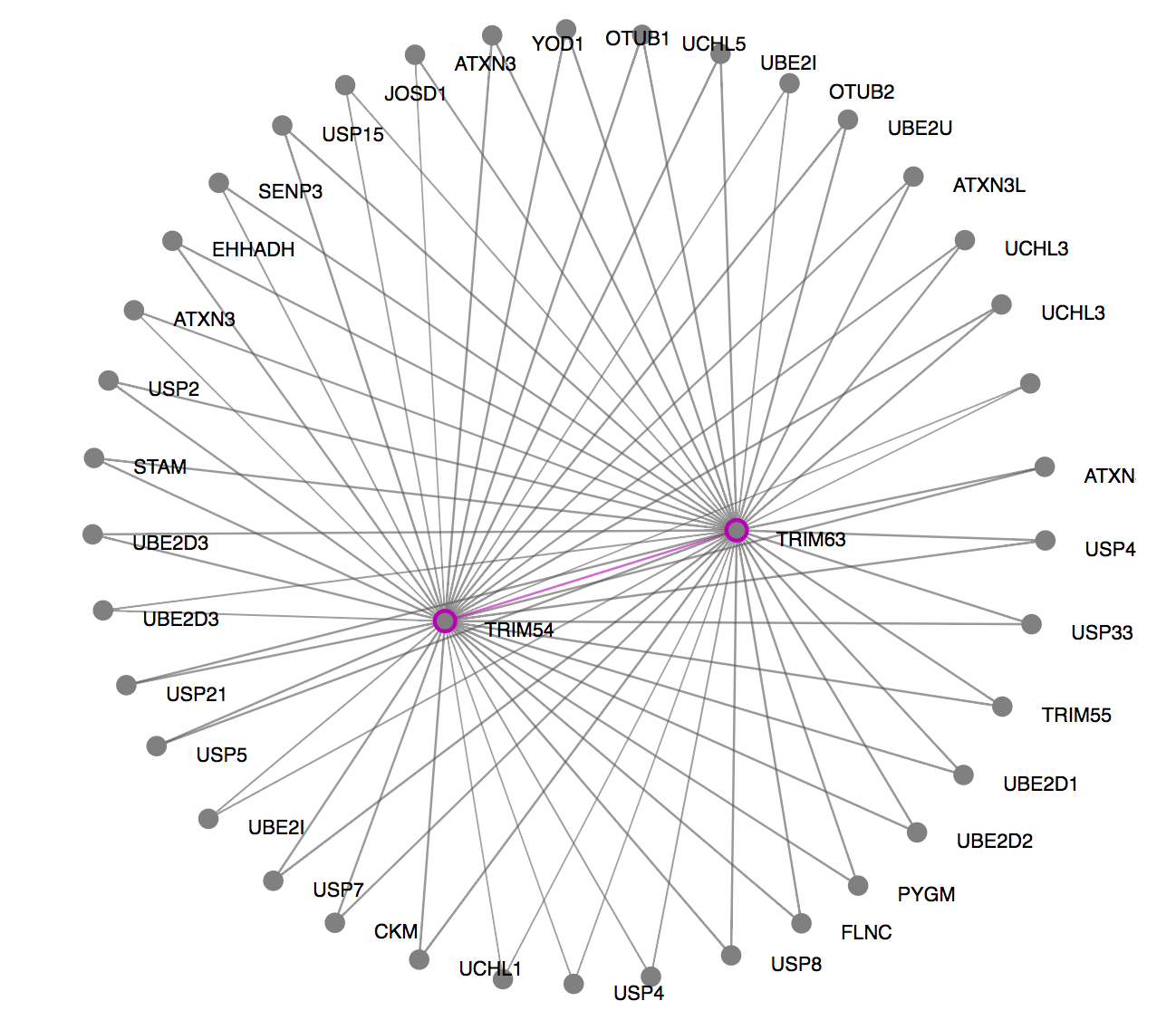
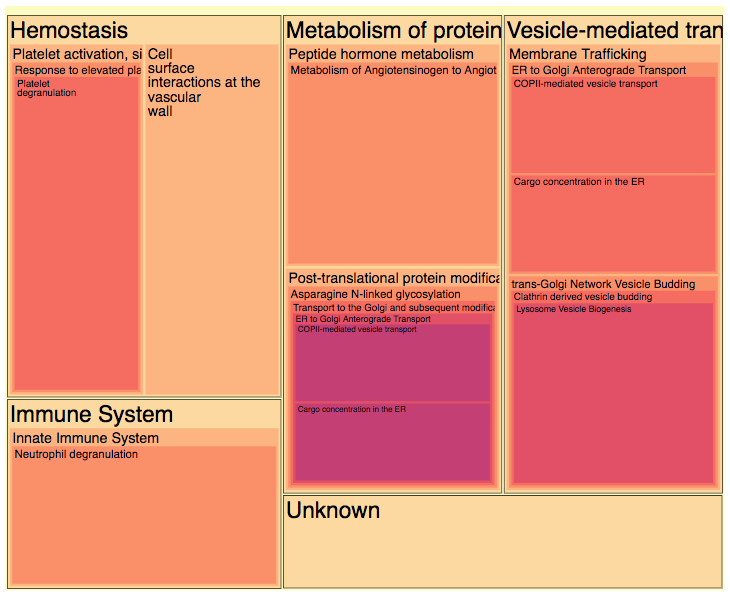
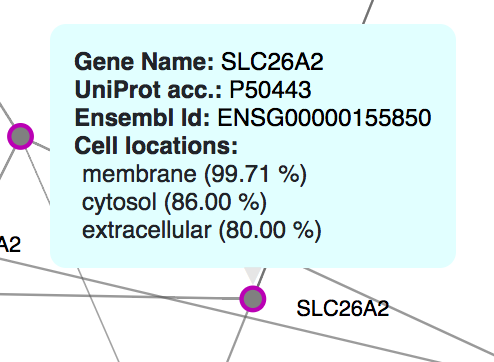
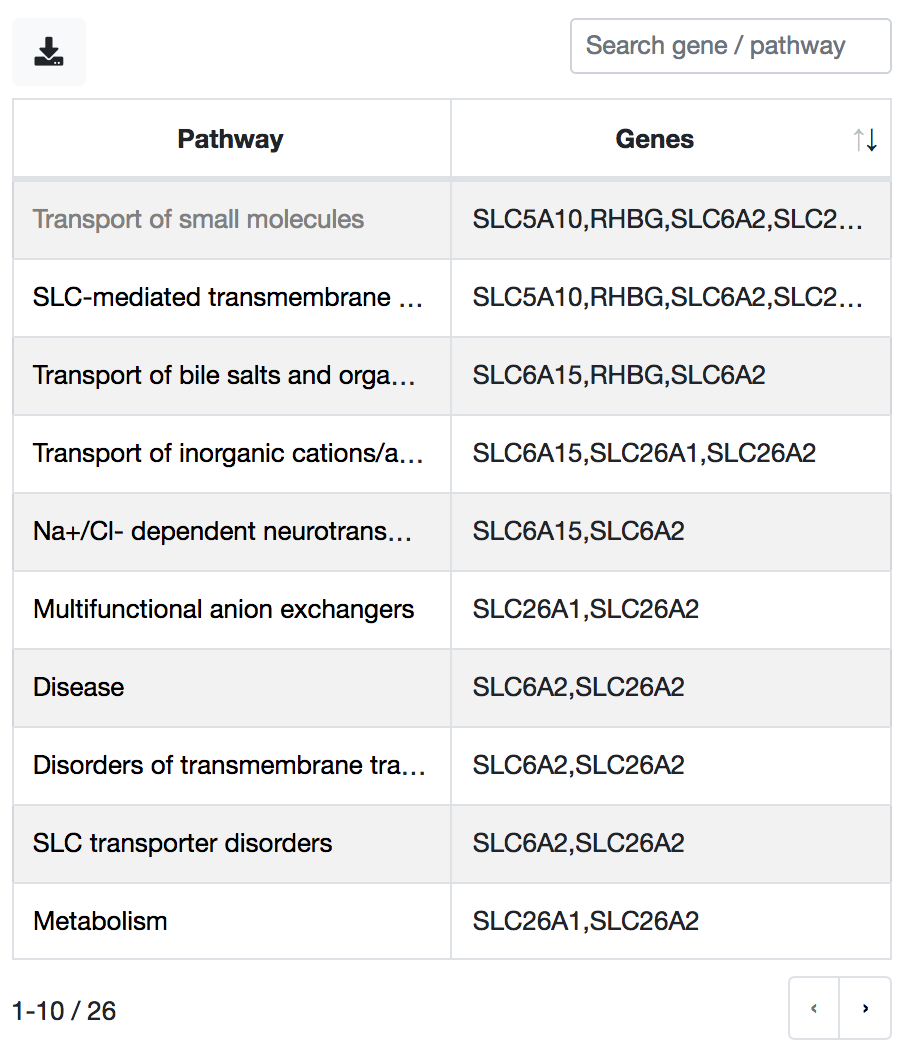
Once you click on the gene module link, ORVAL offers PPI, cellular location and molecular pathway information as a starting (and definitely not exhaustive) point to understand the relevance and interactions of genes in-silico. For an explanation of these graphs, consult the Oligogenic gene module, PPI network and Pathway information sections of our Documentation.
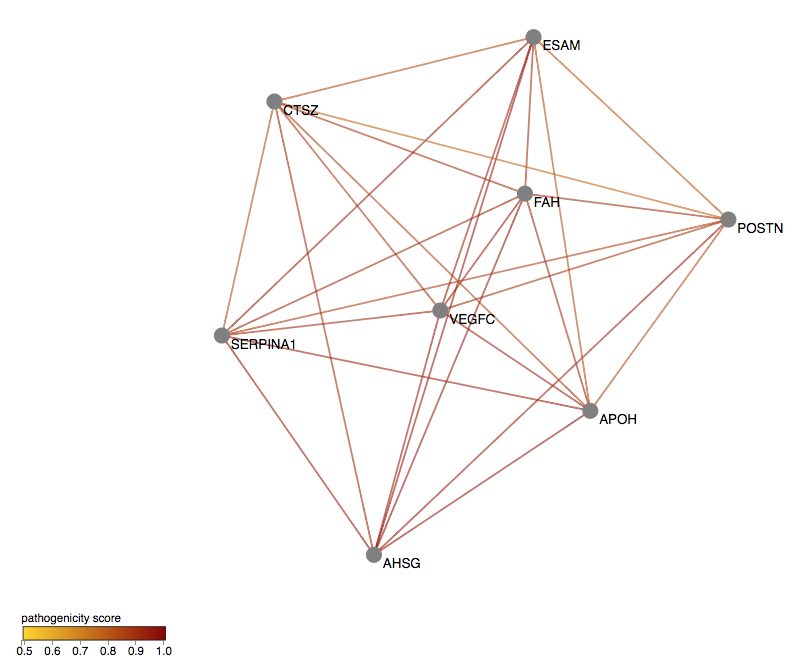
Are the genes in your module related in terms of their PPIs? In the PPI network (the first picture in this example) you can see whether the proteins of this module (purple nodes) directly interact (purple edges) or indirectly interact with one external protein in between, which are depicted as grey nodes and are linked with grey edges. In this example, the proteins of your module TRIM54 and TRIM63 directly interact, but also indirectly interact with several other external proteins found in the comPPI database.
Are the genes in your module involved in similar molecular pathways? You can consult the Reactome pathway treemap and the pathway table to find genes involved in similar pathways (the second picture in this example).
This is a starting point for you to explore the relationships between the genes present in the selected module. Based on this information, you can start limiting the results to the gene pairs that seem to be most relevant.
- 4. Explore the variant combinations of the selected gene pairs

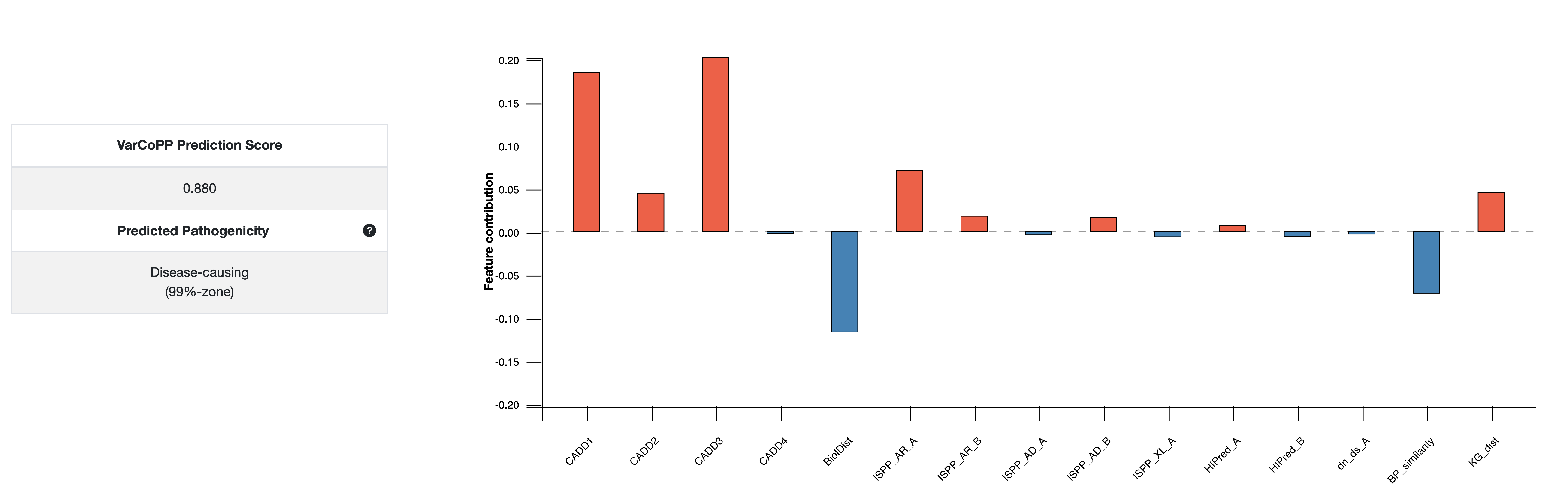
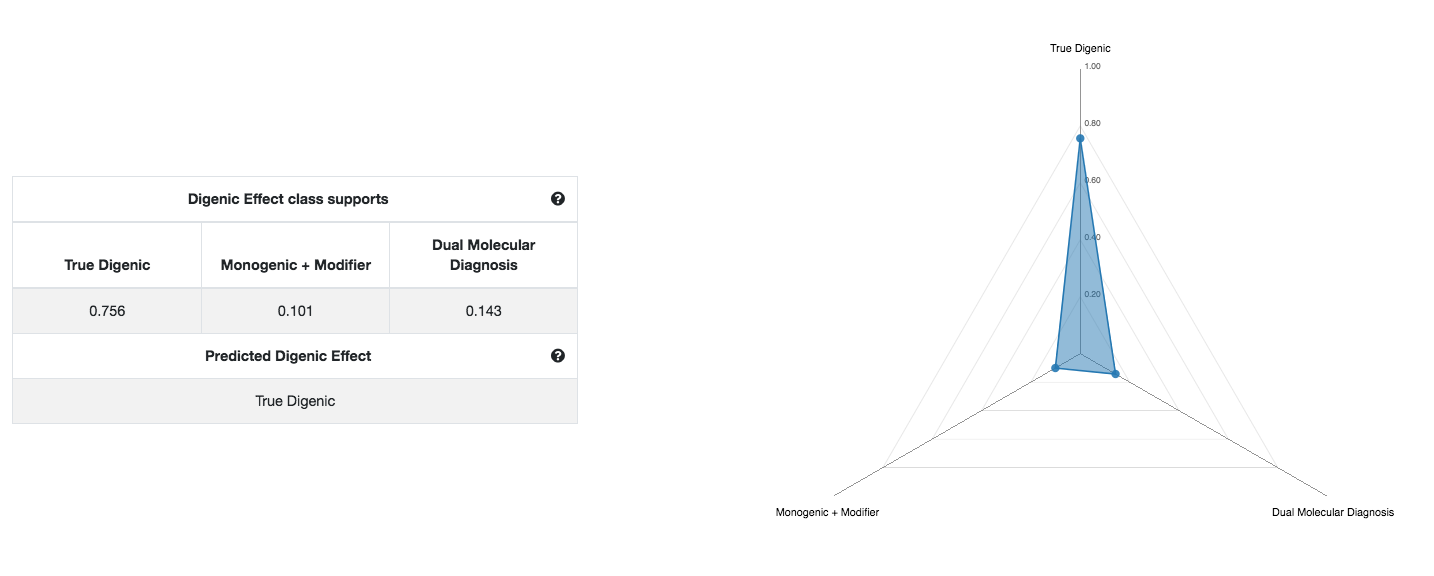
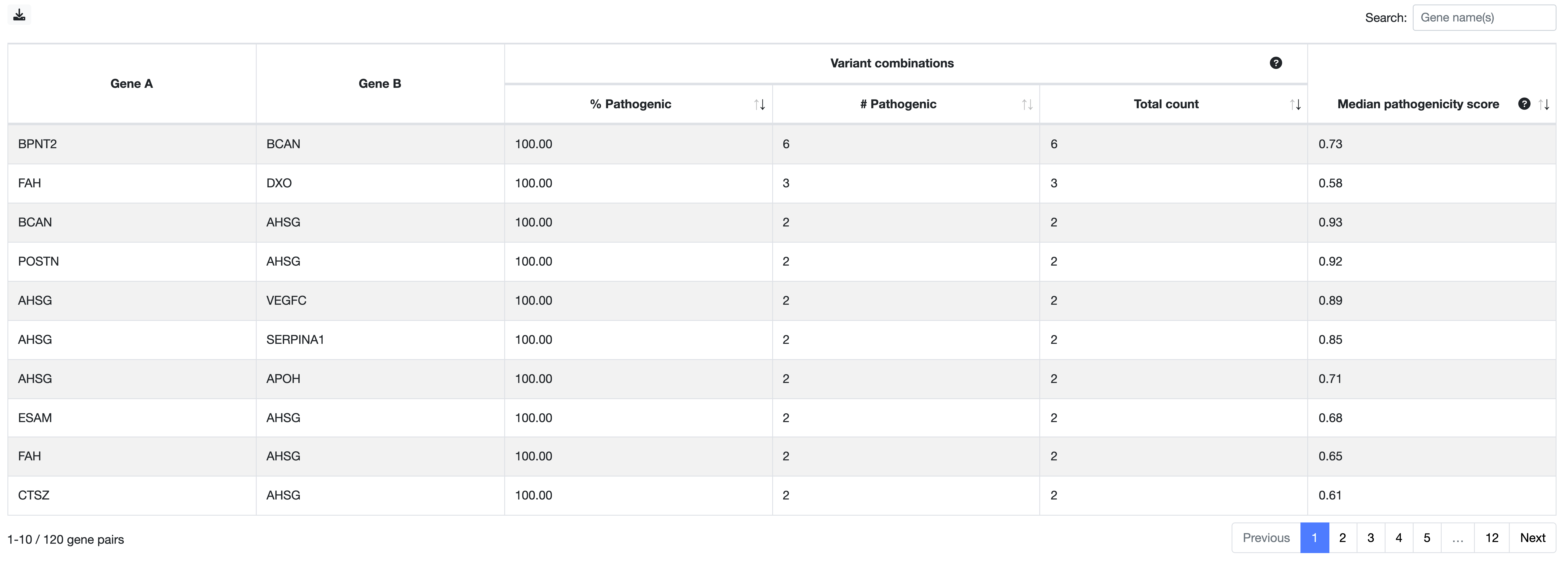
You can now go back to the digenic variant combinations Summary Table (first picture) and the Swarm plot, and explore the variant combinations that are linked to the gene pairs that you have selected based on the previous steps. Especially if multiple variant combinations are linked with the same gene pair, it will be interesting to further explore their predictions to see which are relevant. A summary statistics for each gene pair is also available at the Gene pair ranking table of the Main Results page.
Once you find a combination of interest, you can click on it in the Summary Table (second column) or in the Swarm plot, and you will be directed to a page with information specific for that combination. There, you can first see a summary of the variants and the predictions of both VarCoPP and the Digenic Effect predictor.
You can explore the Feature preference boxplot of VarCoPP (second picture). How do the features vote for either the disease-causing (features in red colour), or neutral (features in blue colour) class? The more these feature preferences deviate from zero, the stronger their vote for a particular class is. In this example, we see that the CADD score of the variant alleles of gene A (CADD1 and CADD2) vote the strongest for the the disease-causing, whereas the CADD score of the first variant allele of gene B (CADD3), as well as its recessiveness probability (RecB) vote the strongest for the neutral class. You can further evaluate the values of these features and why they tend to vote for either class in the Annotations section of that page.
You can further get an indication of the Digenic Effect of that particular combination, i.e. whether it has a True Digenic, Monogenic gene plus Modifier or Dual Molecular Diagnosis effect (third picture). These predictions are indicative and further inspection or confirmation is required. In this example, the variant combination has a very strong prediction for the True Digenic class.
The information in this page can help you evaluate whether the specific variant combination seems relevant for your analysis.
- 5. Examine the relevance of selected gene pairs and combinations for the phenotype
Do the selected gene pairs and variant combinations make sense to you as a clinical researcher and are they in accordance or could they explain the patient's phenotype? At this step, you have a filtered set of gene pairs and variant combinations that could be potentially relevant, based on in silico information.
- 6. Optional: Repeat steps 1-5 with less strict criteria
If at this point the information seems incomplete or you still do not obtain promising results, you could lessen the strictness of your criteria and repeat the steps 1-5. For example, if you have selected only gene pairs with combinations falling into the 99%-confidence zone, you could now also allow gene pairs with combinations falling also into the 99%-confidence zone. Furthermore, if combinations falling into these confidence zones are not available or they do not seem convincing, you can try to include all gene pairs and variant combinations predicted as disease-causing.
- 7. Explore familial and functional evidence
The previous steps provide a way to limit your analysis to those variant combinations that seem to be more relevant or more promising for further research and experiments. ORVAL cannot, in any way, provide a definite way for diagnosis or medical advice. Further evidence is needed to show whether they can indeed be True Positives based on segregation analyses and functional experiments.
Browser compatibility
You can find below which browsers are suitable for ORVAL based on your operational system:
| OS | Version | Chrome | Firefox | Microsoft Edge | Safari |
|---|---|---|---|---|---|
| Linux | Ubuntu 20.04 | 100.0.48 | 99.0 | N/A | N/A |
| MacOS | Monterey | 100.0.48 | 99.0 | N/A | 15.4 |
| Windows | 10/11 | 100.0.48 | 99.0 | 100 | N/A |
Frequently Asked Questions
If answers to your questions are not provided in this section and no information about your question is mentioned in the Documentation page, you can contact us.
In general, we highly recommend the use of variants from up to 300 genes, as well as the application of the variant filtering procedure that is provided with ORVAL, in order to limit the amount non-relevant combinations that will be tested.
Based on our server testings, you can either copy-paste a variant list of up to 80000 variants or upload a VCF file of size up to 50 MB. You can consult the Input Data section of our Documentation page for more details.
No, the analysis should be restricted to a single individual only, as ORVAL creates all possible variant combinations from your variant list assuming that they belong to the same person.
If you want to analyse multiple patients, you should separate their variants in different files and explore them individually with ORVAL.
ORVAL accepts different types of variant format for the insertions and deletions, involving dashes or not.
You can consult the Variant Types section in the Documentation page for a detailed explanation.
If there is no specific error message that explains the problem, you can follow these steps:
- Check if your internet connection is running smoothly.
- Re-try your data submission.
- Check if your input data is correctly formatted. You can consult the Input Data section in our documentation page for a detailed explanation on the correct format for your variant submission.
If you have checked the previous steps and you still experience issues, you can send us an email, providing also the Job Id you have obtained during the submission.
For server monitoring purposes we allow every user (based on their IP address) to run maximum 5 different data analyses at the same time. In case you exceed this number, you have to wait until at least one of the running jobs is finished to launch a new one. You can consult the Job Submission section of the Documentation page for more details.
The VCF file you have uploaded is not correctly formatted and ORVAL cannot parse it. Make sure that your file contains the header line
(#CHROM POS REF ALT etc...) and tab-delimited columns with the CHROM, POS, ID, REF, ALT, FORMAT, SAMPLE_NAME columns.
You can consult the
VCF file specification section of the Documentation page for a detailed description
on how to properly format your VCF file.
ORVAL creates all possible di-allelic, tri-allelic and tetra-llelic variant combinations between any gene pair present in your data, including heterozygous compound variants in one of the two genes. Tetra-llelic combinations with four heterozygous variants (two in each gene) are not created, as these combinations were absent from our training set.
For a detailed explanation, you can consult the Creating digenic combinations section of our Documentation page.
You can check the following steps to explore possible solutions:
- Check whether the selected genome version is correct. ORVAL can annotate variants using the GRCh37/hg19 or GRCh38/hg38 genome version, see the Genome version section of the Documentation page for more details.
- Try to relax your variant or gene filtering options, especially the option for removing intronic and synonymous variants.
- Ensure that you have more than one genes present in your data. ORVAL makes variant combinations between gene pairs, so it requires the presence of at least two different genes in your data.
- If you have submitted a small number of variants, these variants may have been excluded from the analysis during the annotation process. For example, the CADD score for those variants may be missing, and as this score is important for the predictions, variants without an annotated CADD score are excluded from the analysis. A detailed list of all possible cases where your variants may be excluded is presented in the Variant Exclusion section of the Documentation page
If you have checked the previous steps and you still experience issues, you can send us an email, providing also the Job Id you have obtained during the submission.
- Please check the variant and/or gene filtering options you have selected during your submission, as these play an important role on the absence of some of your variants in the analysis. The variant filtering options offered by ORVAL are automatically pre-selected during the submission, unless you unselect them.
- Make sure that the variant information and format you provided is correct for all variants. In case you copy-pasted a variant list using the box panel, make sure that the zygosity values are not misspelled (Heterozygous or Homozygous zygosity values are accepted), see also the tab-delimited variant list section in the Documentation page.
- Otherwise, some variants may have been excluded from your analysis during the data annotation process. You can consult the Variant Exclusion section of our Documentation page for a detailed description of such cases.
For more information regarding our filtering options and the data annotation process, you can consult the
Data Filtering and Annotation
section of our Documentation page.
This can happen for the following reasons:
- The gene symbol present at the gene panel file is not the official HGNC gene symbol. During annotation we only use the official HGNC gene symbols, and thus, we will display this symbol instead of the one that you provided.
- The position of the variant for that gene overlaps with other genes. In this tricky situation, we have to choose one gene to continue with predictions.
We have developed a set of priority rules for that (see the Gene annotation
section). Therefore, there is a chance that we have assigned this variant to another gene instead. Unfortunately, it is not possible to re-assign it
to your favourite gene manually and we can potentially do changes in our database for that only during major ORVAL updates. Please note that you shouldn't change that gene name for the variant in the results,
as some prediction features are gene-specific.
If you think that this mapping is definitely wrong, you can send us an email.
You can see a network in the Results page only if there is at least one candidate disease-causing variant combination predicted with VarCoPP, see for more details the Network navigation section in the Documentation page.
In any case, you can still explore all variant combinations in the Digenic predictions of the Results page.
You see an external protein in the PPI network only if it links two proteins of your selected module with a direct protein-protein interaction. External proteins that are connected with your module proteins with higher degrees of interactions are not shown. You can consult the PPI network exploration section in the Documentation page.
You can see the Digenic Effect prediction of a variant combination on their corresponding Digenic Combination page. You can get directed to this page by clicking on a variant combination either in the Digenic Combinations Overview table or on the Swarm plot.
Please note that you will only see a Digenic Effect prediction if the variant combination is predicted as pathogenic with VarCoPP.
We store the results of your data submission for 7 days, so that you can re-access the corresponding Result pages. After this period, all data is deleted.
We do not store email addresses that you may have provided to us during the data submission. However, we track general user traffic information (e.g. IP addresses) for job monitoring purposes (e.g. restricting the number of parallel submissions from the same ID address).
For a detailed explanation of our Data Privacy procedures, you can consult the Data privacy section of the About page.